Telluride Engineering is skilled in the life sciences industry. With our team’s background in sterile products and vaccine production, we bring this knowledge and experience to all projects from process engineering, control systems and packaging and serialization. We further leverage our background in process safety to take a risk-based approach to tough life science design challenges.
–
Data Integrity

We offer data integrity services, including design of automated manufacturing systems, system configuration, and customization of platforms, software, workstations and 3rd party equipment to meet 21 CFR Part 11 data integrity requirements.
This can be particularly challenging for lab or other COTS (commercial off the shelf) equipment. We have experience in lab software validation and wraparound OS configuration to meet data integrity and validation requirements.
–
Computer System Validation
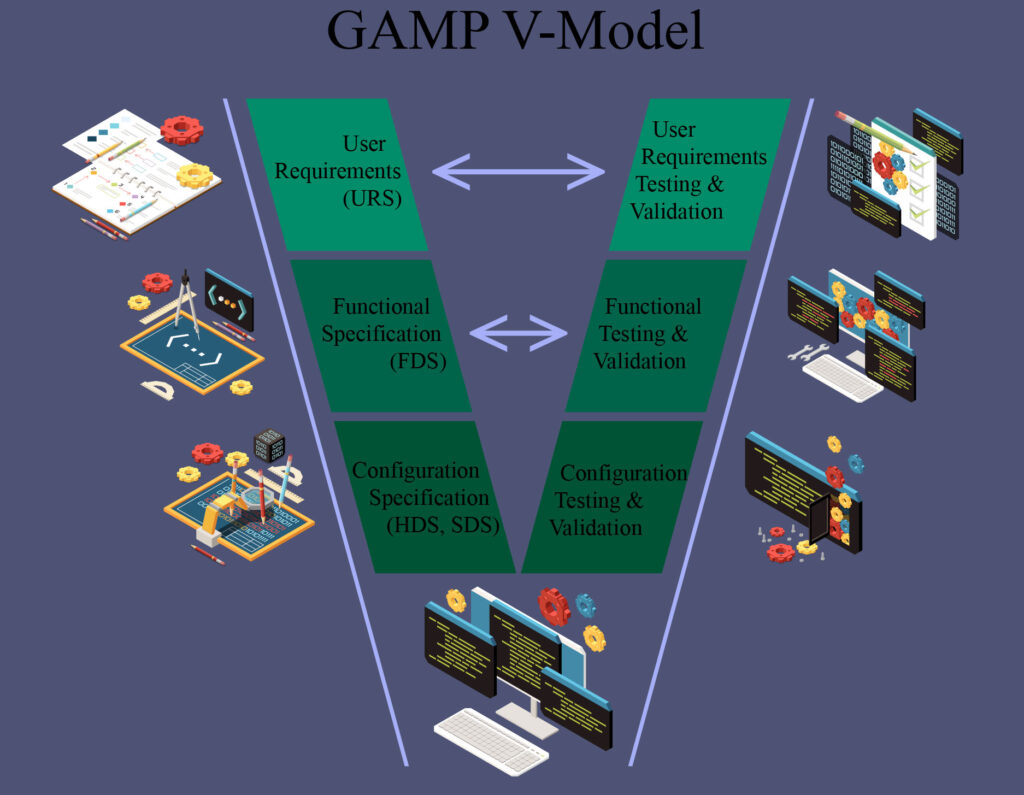
We have extensive experience in the computer system validation process, and can provide a wide array of documentation and validation services. This can be for new facilities, conversions, or any other change requiring validation.
We can provide based documents to be executed by the final owner, or we can train on your standard operating procedures and directly integrate to you validation team and directly execute the documents..
We can author any documents required in tandem with the final owner, such as user requirement specification (URS), functional design specification (FDS), hardware/software design specification (HDS/SDS), installation qualification (IQ), operational qualification (OQ), performance qualification (PQ), standard operating procedures (SOP) or any other document required.
Aseptic Manufacturing

We have a background in aseptic manufacturing, with our chief engineering having worked in engineering operations for a filling, formulation and lyophilization plant. This provides us with extensive knowledge of what challenges the owner will face during the operating stages of the plant, and what to design for.
We can also provide operational insights, whether for a capital project upgrade, or for day-to-day GxP operations needs.
Process and Controls Engineering
Our process and safety knowledge directly inform our strategy about ensuring patient safety and process conformance. We have design capabilities in controls system design, process piping design and other high-hazard or high-consequence realms. We can design for process in both GxP and non-GxP areas throughout the plant.
Read more on our other pages, linked below:
